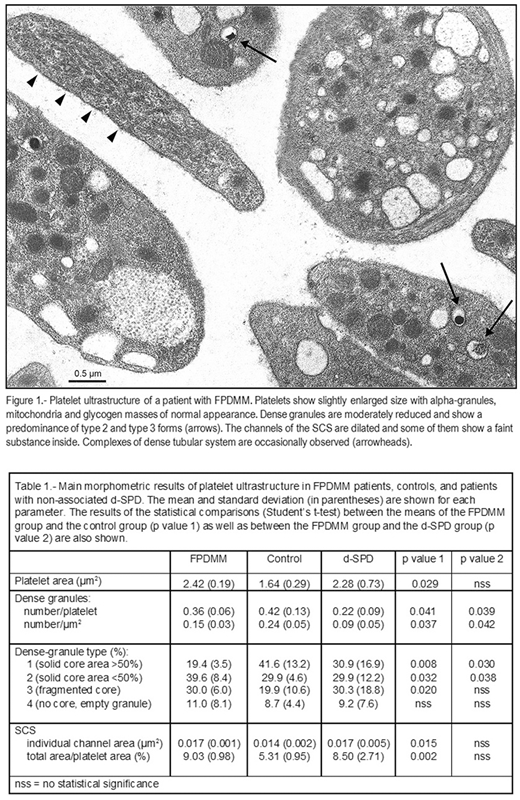
The familial platelet disorder with associated myeloid malignancy (FPDMM) is an autosomal dominant platelet disorder, caused by germline RUNX1 mutations, with predisposition to develop hematologic malignancies, especially acute myeloid leukemia. In many of the FPDMM families reported, the platelet defect was a delta-storage-pool disease (d-SPD) which can also be found without leukemia propensity. However, it has not been studied whether the two types of d-SPD have a common nature. Platelet ultrastructure, previously very little studied, may be one of the aspects to be analyzed to solve this question.
We analyzed the ultrastructural characteristics of platelets in 5 members of a family with FPDMM. The family included three generations and all affected members had a RUNX1 deletion: chr21:36349450-36572837 (Rio-Machin et al. Nat Commun 2020;11:1044). None of the patients studied had developed leukemia at the time of the platelet study. We compared the results with those of 24 patients with d-SPD non-associated with leukemia and with those of 15 healthy individuals.
Platelets were processed by transmission electron microscopy by standard methods. On the electron micrographs, morphometric analysis of the following structures was performed: 1) Platelets: size and shape, 2) Intraplatelet corpuscular structures (dense granules, alpha granules, mitochondria, lipid droplets): size and number (per platelet and per square micrometer of platelet area), 3) Surface-connected canalicular system (SCS): mean area of individual channel sections and mean percentage of the total SCS area with respect to the platelet area, 4) Glycogen masses: total area with respect to the platelet area. The morphological traits of the platelets and organelles measured above were also evaluated and the dense granules were classified into 4 different types depending on the appearance of their solid core (Weiss et al. Br J Haematol 1993;83:282). The dense tubular system and other ultrastructural characteristics were evaluated by morphology only.
The main features of the platelet ultrastructure in patients with FPDMM were (Fig 1, Table 1): 1) slight increase in platelet size while preserving the discoidal shape, 2) moderate reduction in the number of dense granules, which showed a reduced proportion of type 1 granules (with the solid core occupying more than 50 % of the granule) and an increased proportion of type 2 and type 3 granules, with a solid core reduced or fragmented respectively, 3) marked increase and dilatation of SCS with some elements filled by a substance of unknown origin, 4) moderate increase in dense tubular system with occasional complex formation.
The platelet ultrastructure was similar to that described in the non-associated d-SPD group (Pujol-Moix et al. Haematologica 2000;85:619) although there were some differences (Table 1): in FPDMM platelets the dense granules were less reduced but more dysmorphic, and the SCS, equally dilated, contained a substance that was not observed in the d-SPD platelets.
Given that all the findings described belong to the same family, it would be necessary to evaluate the platelet ultrastructure in additional families and extend the study to other characteristics of d-SPD. Only in this way, it would be possible to know to what extent the platelet defect of FPDMM and that of non-associated d-SPD shared a pathogenic mechanism.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.